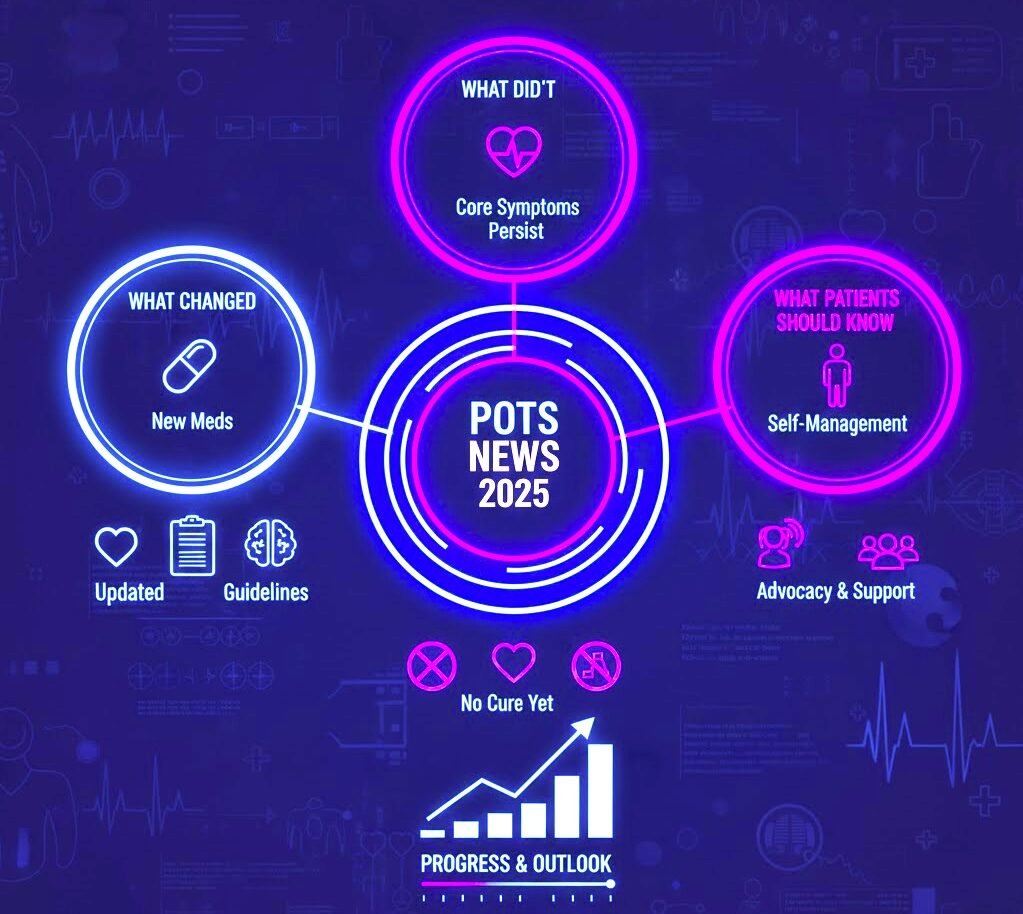
A Clear Look at the Most Important POTS Updates of 2025
For people living with POTS, 2025 brought a mix of meaningful progress, steady trends, and ongoing challenges. While there were no dramatic breakthroughs or new FDA‑approved treatments, several developments shaped the year in ways that matter for patients, caregivers, and clinicians.
This roundup brings together the biggest POTS news of 2025—what changed, what stayed the same, and what these updates mean for the future of POTS care.
What Changed in 2025
A Promising Study on Ivabradine
One of the most notable updates of 2025 was a small but meaningful study showing that ivabradine—a heart‑rate–lowering medication—may significantly reduce symptom burden for POTS patients.
Do you use a walker or other mobility aid for independence?
The study found improvements in:
- Standing heart rate
- Symptom severity
- Daily functioning
While the study was small, it added rare clinical evidence to a medication many patients and clinicians have used off‑label for years. It also highlighted the need for larger trials and more research into heart‑rate–targeting medications.
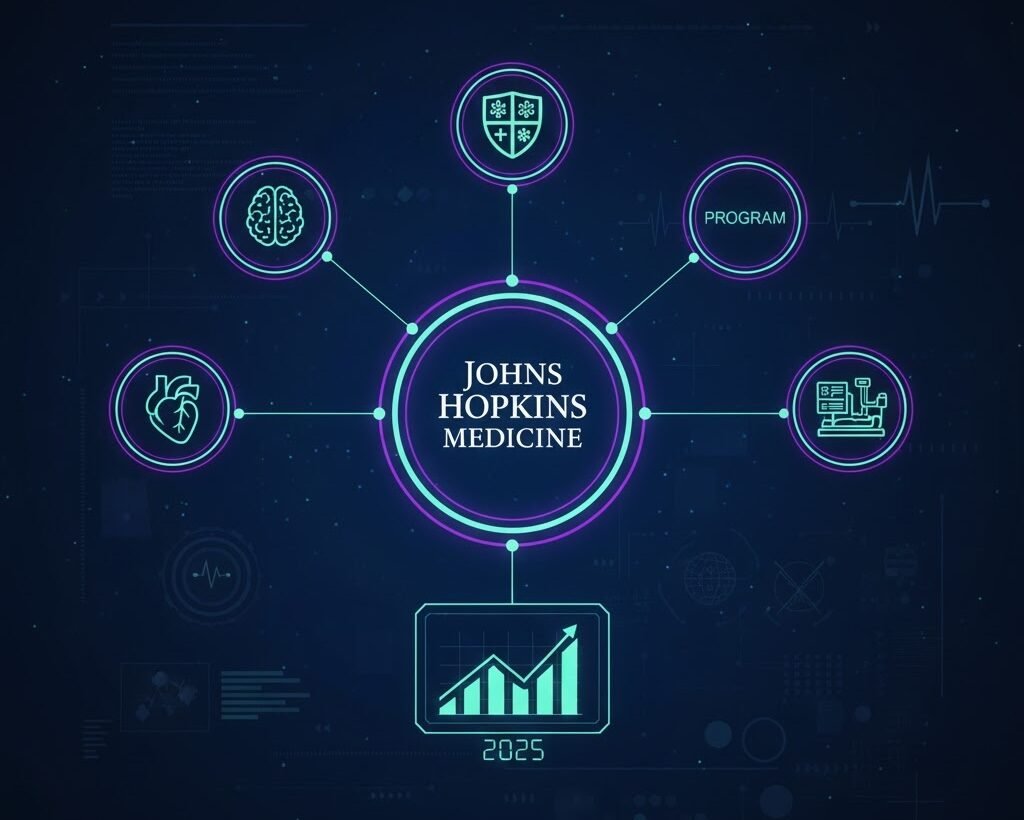
Expansion of the Johns Hopkins POTS Program
Another major development was the expansion of the dedicated POTS program at Johns Hopkins. This expansion strengthened their multidisciplinary “medical home” model, offering coordinated care for patients with complex symptoms and long diagnostic journeys.
The expansion reflects:
- Growing recognition of POTS as a legitimate medical condition
- Increased demand for specialized autonomic care
- A shift toward multidisciplinary evaluation and support
This was one of the clearest signs that major institutions are taking POTS more seriously.
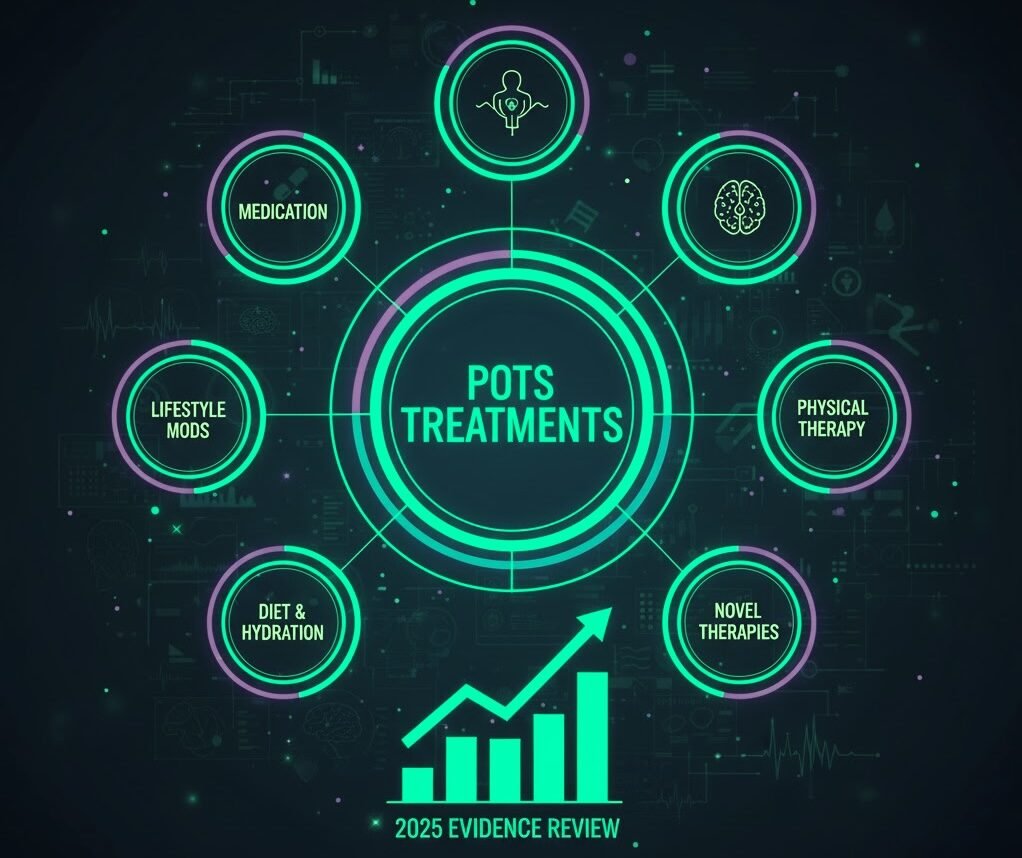
A Comprehensive 2025 Evidence Review
A systematic review published in 2025 evaluated the current state of POTS treatments. While it did not introduce new therapies, it clarified which strategies have the strongest support and where evidence remains weak.
Key takeaways included:
- Exercise‑based rehabilitation remains the most supported intervention
- Non‑pharmacological strategies are foundational
- Medication evidence is limited and inconsistent
- More research is urgently needed
This review helped bring clarity to a field often shaped by small studies and clinical experience.
What Didn’t Change in 2025
No New FDA‑Approved Medications
Despite increased awareness and research interest, 2025 did not bring any new FDA‑approved medications for POTS. Most treatments remain off‑label, and medication responses continue to vary widely.
No Major Diagnostic Breakthroughs
Diagnostic criteria for POTS remained unchanged. While awareness improved, there were no new diagnostic tools or standardized testing protocols introduced in 2025.
No Large‑Scale Clinical Trials
The year did not produce any large randomized controlled trials—the kind needed to transform treatment guidelines. Most studies remained small, observational, or preliminary.
No New National Guidelines
There were no new national or international guidelines published for POTS diagnosis or treatment. Clinicians continue to rely on existing consensus statements and clinical experience.
Why These Updates Matter for Patients
More Recognition, Even Without Breakthroughs
The expansion of major programs and publication of new reviews signal that POTS is gaining visibility in academic medicine. This recognition helps reduce stigma and encourages more clinicians to learn about autonomic disorders.
Clearer Understanding of What Works
The 2025 evidence review reinforced the importance of foundational strategies such as:
- Hydration and electrolytes
- Compression garments
- Structured exercise programs
- Pacing and energy management
These strategies remain central to POTS care and continue to show the strongest support.
Hope for Future Research
The ivabradine study, while small, demonstrates that researchers are actively exploring treatment options. Each new study helps build momentum for larger trials.
Better Access to Multidisciplinary Care
The Johns Hopkins expansion highlights a growing trend toward coordinated care models. As more institutions adopt similar approaches, patients may experience shorter diagnostic journeys and more comprehensive support.
What Patients Should Know Going Into 2026
POTS Research Is Growing
While progress is slow, the number of studies, reviews, and clinical programs continues to increase. Awareness is higher than ever, and more clinicians are becoming familiar with autonomic disorders.
Foundational Strategies Still Matter Most
Hydration, salt intake, compression, and structured exercise remain the most supported interventions. These strategies continue to form the backbone of POTS management.
Medication Options Are Still Evolving
Ivabradine’s 2025 study adds to the conversation, but medication evidence remains limited. Patients should expect individualized approaches rather than one‑size‑fits‑all solutions.
Multidisciplinary Care Is Becoming More Common
Programs like the one at Johns Hopkins are setting a new standard for POTS care. As more institutions follow suit, patients may see improved access to coordinated evaluation and support.

GnarlyTree | DIET AND EATING
The Role of Yogurt and Probiotics | POTS Guide to Gut Health
One area with POTS that doesn’t get nearly enough attention is gut health—yet the digestive system plays a major role in daily stability, energy, and overall comfort. Yogurt and probiotics can be powerful tools for...
Frequently Asked Questions
What was the biggest POTS news of 2025?
The ivabradine study and the expansion of the Johns Hopkins POTS program were the most significant updates.
Did any new medications get approved for POTS in 2025?
No, there were no new FDA‑approved medications for POTS this year.
Did research show any new treatments?
No new treatments emerged, but existing strategies were evaluated more thoroughly.
What did the evidence review say about exercise?
It confirmed that structured exercise remains one of the most supported interventions for POTS.
Did diagnostic criteria change in 2025?
No, diagnostic criteria and testing protocols remained the same.
Why is the Johns Hopkins expansion important?
It reflects growing recognition of POTS and provides more access to coordinated, multidisciplinary care.
Are medications still used off‑label?
Yes, most medications used for POTS remain off‑label, including those studied in 2025.
Is research increasing?
Yes, awareness and research interest continue to grow, even if progress is gradual.
What should patients focus on in 2026?
Foundational strategies, symptom tracking, and staying informed about new research.
Final Thoughts
2025 may not have delivered dramatic breakthroughs, but it brought meaningful progress in recognition, research clarity, and clinical support for POTS patients. The ivabradine study offered new hope, the Johns Hopkins expansion strengthened access to specialized care, and the evidence review helped clarify what treatments show the most promise.
For a condition as complex as POTS, progress often comes in steps rather than leaps. This year’s developments show that the field is moving forward—slowly but steadily—and that patients are gaining more visibility, more support, and more reason to hope for continued advancements in the years ahead.
Do you use a walker or other mobility aid for independence?